We briefly mentioned the structure of the atom in previous classes. Today we will attempt to understand this structure more fully, including how atoms emit electromagnetic energy ("light"), and how astronomers use that light to unravel the nature of distant objects. First we return to the basic structure of the atom. All of the elements in nature are composed of just three types of subatomic particles: protons, electrons and neutrons. Protons and neutrons stick together to form the nuclei of atoms (nuclei is the plural of nucleus). And the electrons orbit around the nucleus sort of like planets orbiting the Sun.
Electrons have a negative charge while protons have a positive charge. The attraction between the negative charge of an electron, and the postive charge of the proton, is what keeps the electrons in orbit around the nucleus. Instead of gravity, it is the "electrostatic" charge attraction that holds atoms together. It is informative to look at the masses of these two particles. A proton has a mass of 1.6 X 10-24 gm, while the electron has a mass of 9.1 X 10-28 gm. Electrons have a much smaller mass than the proton--this is why they are the particle in orbit--like the planets, the electrons orbit the nucleus because they are the less massive objects. Because of the size of these tiny numbers, physicists use the mass of the proton as the unit when talking about the masses of atoms. In this system, the proton has a mass of "1", and an electric charge of +1 unit, while the electron only has a mass of 1/1836 proton masses (that is a proton is 1,836 times more massive than an electron), and a negative charge of -1 unit.
Neutrons are particles with the same masses as protons, but they do not have any charge--we say they have a "neutral charge", or a charge of "0". Thus, neutrons do not really affect the chemistry of atoms, though they play a significant role in radioactivity. You might think that neutrons are one of those peculiar mistakes of nature, but there is a very good reason for why they exist. If you are interested, you can go to this excellent site to read more about the structure of the atom, the nucleus, and what protons, neutrons and electrons are, and how they interact.
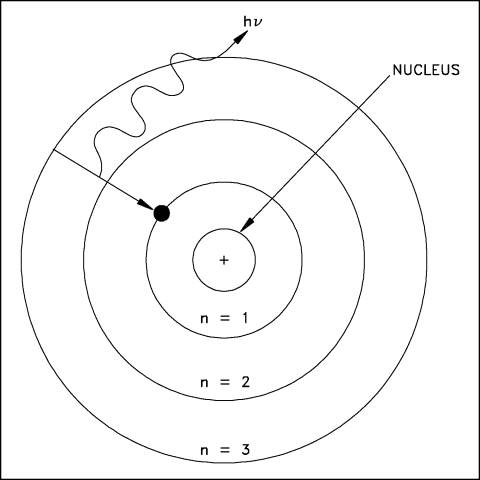
A simple way to visualize the structure of the atom is the solar system model, where we have electrons (planets) orbiting the nucleus (the Sun) in particular orbits. A schematic of this layout for the hydrogen atom is shown below:

In an atom, there are specific "orbits" (n=1, n=2, etc.) in which an electron can actually orbit the nucleus. The rules for determining these orbits are quite complicated, and we will not discuss them here (it is the physics of "quantum mechanics" that is needed to fully describe the structure of atoms), but we say that the orbits are "quantized". An electron revolves around the nucleus in a particular orbit. There are a set of orbits in every atom determined by how many protons are in the nucleus, and how many other electrons are in the atom. In the hydrogen atom there is one electron in orbit around the one proton that comprises the hydrogen atom nucleus. As we discussed last class, there can be other types of hydrogen. In the hydrogen isotope deuterium, the proton is joined by a neutron. In the hydrogen isotope tritium, there are two neutrons and one proton in the hydrogen nucleus. Note, however, that the number of neutrons has no effect on the charge of the nucleus, so all of the isotopes of hydrogen act the same way--it is the number of protons and electrons that determine the chemical properties of an atom (giving rise to the periodic table). Thus, water ("H2O") can be made with any of the isotopes of hydrogen and it will still act just like water! Figure 4.7 of the textbook summarizes the terminology of of atoms, here is the part dealing with isotopes:
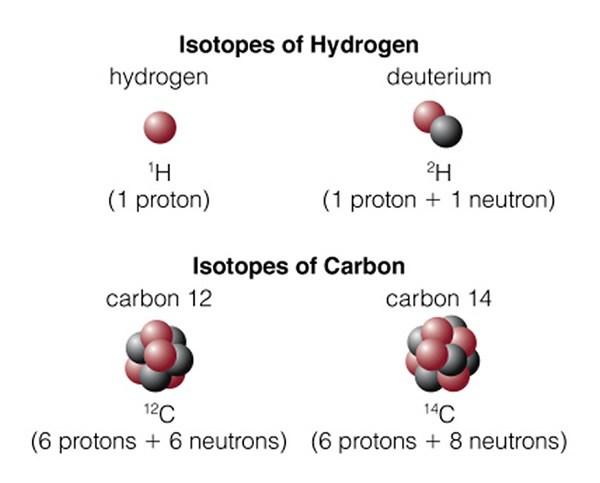
On the top we have normal hydrogen (left) with one proton, and deuterium (right) with a proton and neutron. Larger/heavier atoms like carbon (in the bottom of the figure), have multiple isotopes. Normal carbon is "carbon 12" (12C), while the unstable, radioactive isotope of carbon is "carbon 14" (14C). Normal carbon, 12C, is called that because the mass of the carbon atom is 12 units of proton mass. There are six protons and six neutrons in the carbon nucleus. But the atomic number of carbon is "six", as it is the number of protons (which is the same as the number of electrons, giving rise to the ordering of the elements in the periodic table) in an atom that determines how it will behave:
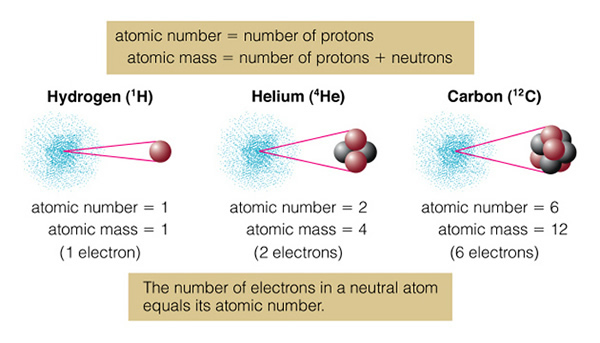
Normally, all atoms are "neutral" (at least on the Earth), that is there are exactly the same number of protons and electrons in the atom. If, however, you remove one of the electrons from the atom, you form an "ion". For example, if carbon normally has six electrons and six protons, it has a neutral charge--it is uncharged (charge of "0"), and we could write this as C0. If we remove one electron from the carbon atom, the nucleus will still have six protons, but now there are only five electrons orbiting the nucleus. This carbon atom now has a charge of +1 (since we took away a -1 charge). We call this atom "a singly charged ion of carbon", and physicists write it as C+1. Astronomers have their own notation for this. Astronomers call neutral carbon "C I" instead of C0, and C+1 as "C II". This helps eliminate having to put in lots of superscripts! What do you think C III means? That's right, it is carbon with two electrons removed (C+2).
Let's return to talking about electrons and orbits. Each of the orbits in an atom corresponds to a certain amount of energy. Electrons with lots of energy are in orbits far away from the nucleus, while electrons with low energy are close-in to the nucleus. If we put a bunch of hydrogen atoms inside a metal box, and chill this box in a very cold freezer, we will find that the electrons in all of the hydrogen atoms will be in the lowest energy orbit. Physicists call this the "ground state". Electrons prefer to orbit close-in to the nucleus--they like the smallest orbit possible. If we now shine a high intensity light on our hydrogen atoms, we will find that some of the electrons move out to larger orbits. What has happened? Well, some of the electrons have absorbed some of the light energy, and by increasing their energy, they have to move out to a more distant orbit. To get back to the ground state, the electron will have to give-up this added energy. How does it do this? By emitting light! In nature, this process is happening all of the time, electrons inside atoms absorb some light, move out to a higher orbit, and, a little while later, turn around and emit that light. Electrons are not very good at holding on to energy--in fact, in the hydrogen atom, the electron will usually only hold onto this energy for a tiny fraction of a second before giving it back. Electrons like to be in the ground state, and not wander too far from home.
What makes this process special is the quantized nature of the electron orbits. As we said, there are only a special set of allowed orbits for an electron. Thus, there are only a special allowed sets of energy for an electron. Each orbit has a particular energy associated with it--we say the energy of an orbit is "quantized". To move from one orbit outwards to another, the electron must absorb exactly the right amount of energy--it cannot absorb more or less, it has to be exactly right (sort of like Goldilocks and the porridge!). The electron must absorb a photon of light with exactly the right amount of energy. Oh, oh, a new word: photon. What is a photon? We have already encountered the electromagnetic spectrum--it runs from low energy forms of light like radiowaves and microwaves, through the infrared and visible (where our eyes work), to higher forms of energy like ultraviolet, X-rays and Gamma-rays. Here is the plot of the electromagnetic spectrum:
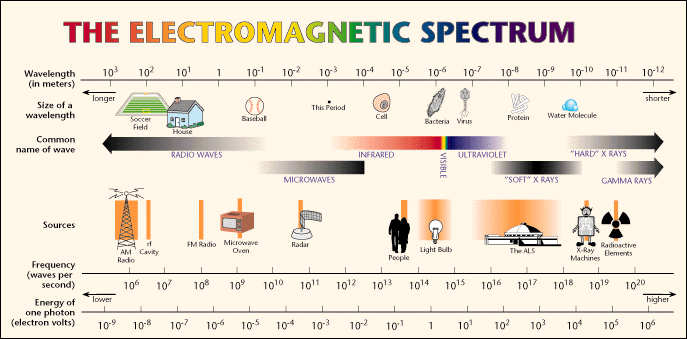
Light is an electromagnetic wave--a small packet of energy that travels through space (and air and glass) as a wave, but it carries energy in a packet we call a "photon", and when a light wave encounters matter, it acts like a particle--the photon--and this particle can collide with electrons. It is a difficult concept to understand. When light is traveling through space, it acts like a wave (just like water waves), and it has a "wavelength":
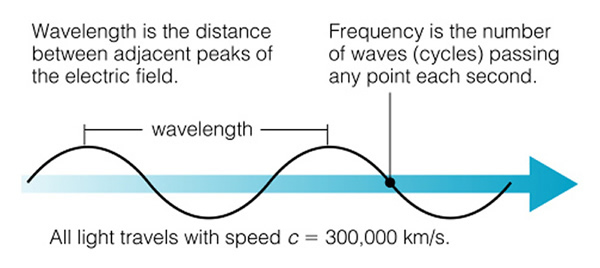
Light (and that includes all kinds of light from radio to gamma-rays) is a wave, and we classify light by its wavelength (Fig. 6.5). The shorter the wavelength, the more energy that type of light has. The longer the wavelength, the less energy the light has (note that the two figures about the electromagnetic spectrum presented here have the direction of high and low energy reversed. Astronomers always put low energy to the right, and high energy to the left--just an oldtime convention, as shown in the next figure 6.5):
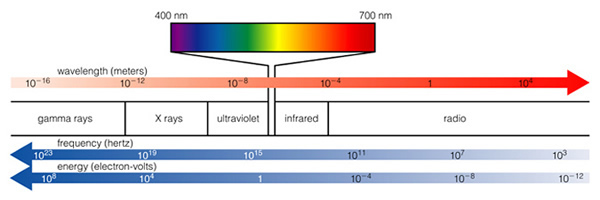
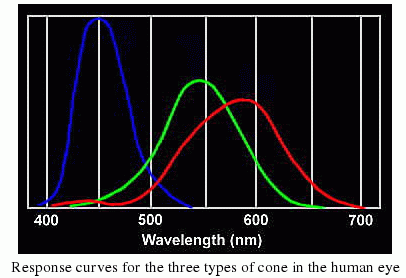
Our eyes are sensitive to visible light, because that is the dominant kind of light emitted by the Sun. Evolution has insured this--it would be hard to see anything if our eyes were sensitive to X-rays, as X-rays from the Sun do not penetrate our atmosphere, and are not easily reflected! Thus, we can't use X-rays, and thus no animals have eyes that are sensitive to something besides visible light (note that some types of snakes have special organs that are sensitive to infrared light--heat radiation--and this allows them to hunt in the dark by detecting the heat radiated by a mouse, while some insects and animals have eyes sensitive to the bluest visible light, called "ultraviolet"). What you need to grasp from this discussion is that light carries energy, and electrons can absorb light, and electrons can emit light. They do this by absorbing or emitting a photon with a certain amount of energy. Here is our model of the atom again (the "Bohr" model, after Niels Bohr):

This diagram shows an electron in a hydrogen atom moving from the n=3 orbit to the n=1 (ground state) orbit by emitting a photon (the squiggly arrow). This photon will have a prescribed (and predictable!) amount of energy. The electron could move back out to the n=3 from the n=1 orbit by absorbing a photon with exactly the same amount of energy as the one it just emitted. Because the energy of the orbits are quantized, the photons emitted by the electrons can only have certain values of energy. This is shown here, a more complicated diagram of the hydrogen atom:
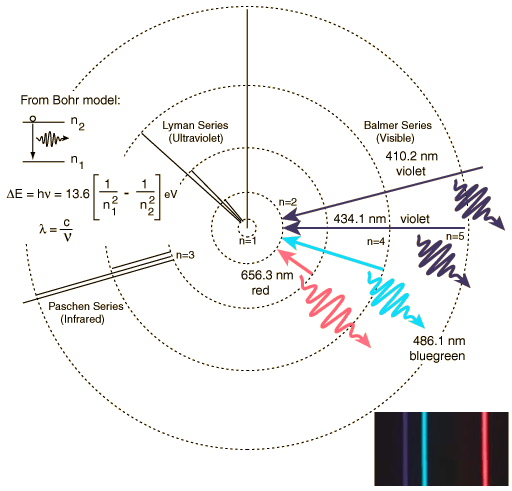
This diagram now shows several "transitions" (that is the movement of electrons between orbits). If an electron moves from the n=3 orbit to the n=2 orbit it emits red photons. If the electron moves from the n=4 to the n=2 orbit, it emits a blue photon. The further out the orbit, the more energy it has, so when it transitions inwards, it releases photons with more energy, and more energy means bluer, and bluer, until you reach the ultraviolet. A bigger transition releases more energy, and more energy means shorter wavelength. We can look at these transitions a different way, instead of thinking of them as circular orbits, we can think of them as being steps. The first few steps are big, but as you go further out in the atom, the steps become smaller and smaller. The following diagram (Fig. 6.8a) is this alternative way to view electronic transitions:
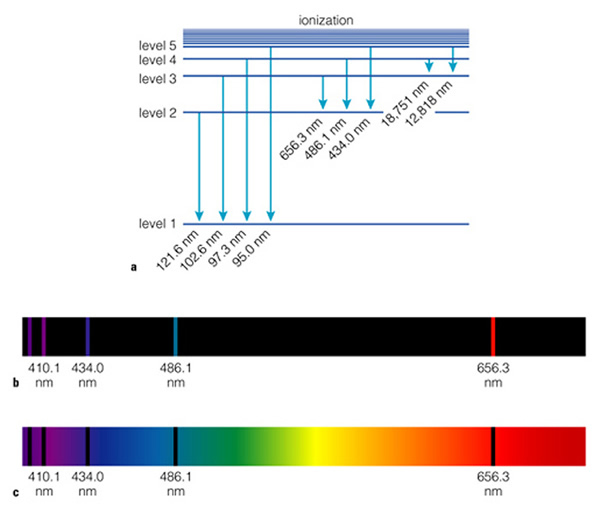
Note that jumping from a higher to a lower "level" (level=orbit!) in the atom releases energy. Jumping from a lower to a higher level requires energy. Thus, we have two kinds of "spectra", emission line spectra (Fig. 6.8b), and absorption spectra (Fig. 6.8c). Note that the "emission lines" are at the same place as the "absorption lines"--they have to be, as the photons that are emitted have identical wavelengths as those that are absorbed. So why are we spending so much time on this? Because each element has a different internal structure, and because of this, the spectrum of each element is unique. Here are three examples (Fig. 6.8):

Just like using fingerprints to identify people, astronomers can use spectra to identify elements! This is the most important tool that astronomers have. By knowing how much energy it takes to create certain spectral lines, we can figure out the temperature of the gas that is emitting this light. Thus, we are also able to measure the temperature of gas using spectral lines. We will learn a bit later that we can use spectra to find out how fast an object is moving. Let's look at the spectrum of the Sun:
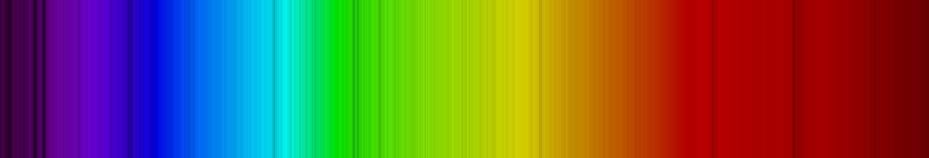
Look at all of those lines! Here is a close-up:

It takes quite a bit of detective work to fingerprint the Sun, and identify all of those lines (but it has been done). You see that the spectrum of the Sun is all absorption lines. Why is that? Well this takes just a bit more explanation, and the relevant diagram is Figure 6.14:
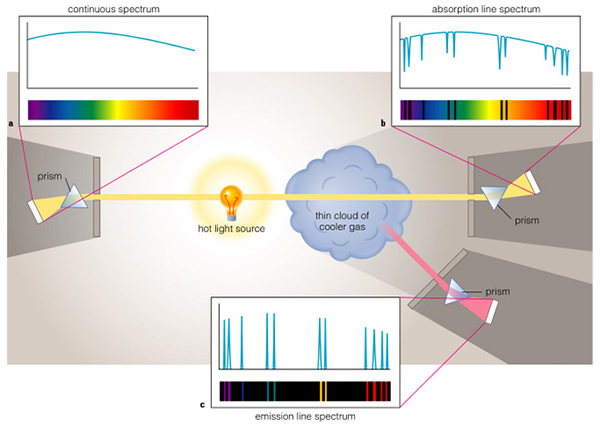
There are three kinds of spectra: absorption, emission, and "continuous" ("spectra" is the plural form of the word "spectrum"). We have encountered the first two already--we need to discuss the continuous spectrum. In hot, dense objects (like inside the Sun, or in the metal filament of a light bulb), some electrons are free to move around. If some electrons are free, however, that means that some of the atoms in the Sun, and in the filament of the light bulb, have positive charges, as they have lost electrons. As these electrons pass by a charged atom (an ion), they can become temporarily stuck in an orbit around that atom, jump between levels and emit photons. But in the meantime, another electron comes zooming by and runs into this electron--it can knock it out of the orbit, and this one now gets trapped in this atom (sort of like musical chairs!). All of this jumping around by the electrons means that there are always a bunch of electrons emitting light. The question you should be asking is "Why isn't the spectrum of a light bulb or the Sun just a bunch of lines?" This is because the nearby ionized atoms can alter the orbits of some of these electrons, and by doing so slightly change the energy levels of those atoms. Because there are billions and billions of nearby atoms, we have billions, and billions of possible orbits. This means that just about every wavelength of light is emitted by a hot, dense object! I know this is complicated, but this is the explanation for why a light bulb emits the full spectrum of visible light, as does the Sun.
But why an absorption spectrum for the Sun? Because, above the hot, dense parts of the Sun, is a cooler, less-dense atmosphere. In this atmosphere, the atoms act normally and can absorb and emit light in the normal fashion. As Figure 6.14 shows, we see an aborption line spectrum because we see a hot continuum source behind the thin, cool atmosphere; we see the lines in absorption. Cool gas absorbs energy.
The only time we see an emission line spectrum is when the gas is very hot, and has a low density. If the gas has a high density, the orbits are distorted and we see a continuum spectrum. But in low density gas, the atoms are further apart, and they cannot change the orbits of electrons in other atoms. In this case we see an emission line spectrum. You will have a lab exercise where you look at the spectra of atoms later this semester.
I want to briefly review atomic structure, as it is the physics of atomic processes that allow us to understand the universe around us. Let's start by remembering the structure of the atom--we have a small nucleus made up of protons and neutrons, with electrons "in orbit" around it. Protons are massive particles and have a positive electrical charge of +1 unit. Neutrons have the same mass as protons, but have no electrical charge. Electrons have a tiny mass (1/1,836 that of a proton), and have a negative electrical charge of -1 unit. It is the opposite charges of the electrons and protons that form the attraction that keeps atoms together. Here is our simplified model of the atom (the Bohr model):

Transitions of electrons between orbits creates spectral lines. An electron in an inner orbit can absorb a photon and transition to a higher orbit, or an electron in a higher orbit can emit a photon, and transition to a lower orbit. Note that these photons have specific, well-determined and precise energies--we say the radiation is quantized. This creates two of the types of spectra observed in nature, an absorption line spectrum, and an emission line spectrum:

The third kind of spectrum is a continuous one, created in hot, dense objects where the nearby electrons and nuclei distort the electron orbits, creating an infinite number of possible orbits, or even partial orbits, that allow for a non-quantized type spectrum with nearly all wavelengths being represented. Light bulbs, stove top burners and stars emit continuous spectra because they are hot and dense. The temperature of the source of the continuous spectrum, however, determines the color of the source. An electric stove-top burner emits mostly red light because it is relatively cool, about 500 K (roughly 400 oF). In fact, a source this cool emits > 95% of its energy in the infrared region of the electromagnetic spectrum. A light bulb filament is quite a bit hotter, with a temperature near 2,700 K, this temperature source also emits most of its energy in the infrared, but enough occurs in the visible to be useful for indoor lighting. The Sun has a temperature of 5,800 K, it emits most of its light in the green/yellow portion of the visual band of the electromagnetic spectrum. Hotter stars emit more blue light than the Sun, while cooler stars emit more red light. We will use this fact to determine the temperatures of stars.
A continuous spectrum has a specific shape, sometimes called a thermal radiation spectrum, and sometimes called a "blackbody spectrum". The following figure (6.13 of the text) shows the wavelength-dependent shape of the thermal spectrum:
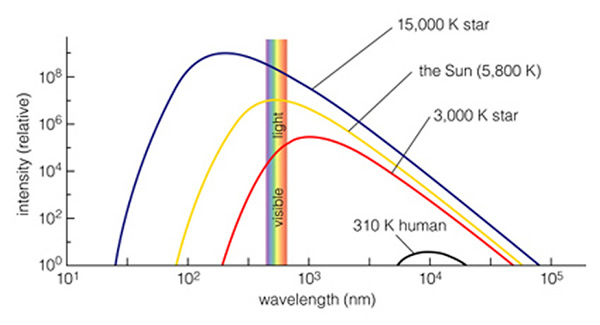
In this figure, the y-axis is a measure of how many photons are coming from the source each second (called intensity). The x-axis is in nanometers (10-9 meters). The hotter the star, the "bluer" its spectrum. The cooler the star, the "redder" its spectrum. Remember that as you transition from yellow, to green, to blue, to ultraviolet, the wavelengths of the light are getting shorter, and the individual photons contain more energy. Thus, by measuring the intensity at two or three wavelengths on the continuous spectrum curve, you can measure an object's temperature! All dense objects that have a temperature above 0 K emit thermal radiation--even humans. Our body temperature is 98.6 oF (310 K), so we emit all of our energy in the infrared (near 104 nanometers).
We briefly encountered nuclear fission when we were discussing radioactive decay. Nuclear fission is the instantaneous (either natural or induced) splitting of the nucleus of a high-mass element such as uranium. This diagram (figure 15.5 from the text) shows a large nucleus of protons and neutrons splitting into two smaller (daughter) nuclei:
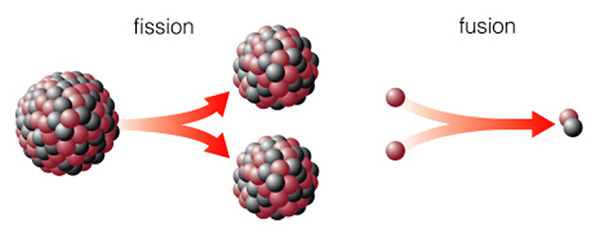
We can use radioactive decay to determine the ages of rocks, and geologists have used this technique to determine the age of the Earth, Moon, and solar system (from meteorites). Nuclear fission can also be used to run a power plant, because when a nucleus splits, it releases energy. Nuclear fission can also be used to make an atomic bomb. Many elements with large nuclei are unstable. The reason these nuclei split is quite technical. The proper way to view it is that the force that holds the nucleus together (the strong nuclear force) only works over very short distances. As a nucleus becomes bigger, the attractive force can just barely hold the nucleus together--the nucleus can be envisioned as a vibrating sphere of jello. Wait long enough (say a "half life"), and the nucleus might spontaneously split.
Or, you can add energy to the nucleus and force it to split. How do you do the latter? If you bombard the nucleus with high energy neutrons and protons, these particles can impact a nucleus, adding energy, and causing it to break apart. This is how both nuclear reactors and atom bombs work. When a nucleus breaks apart, the pieces can then encounter other nuclei, and cause them to split. If there is sufficient unstable material (say plutonium or uranium), and nothing to stop the process, then the result can be a bomb. In a nuclear reactor, rods of a proton/neutron-absorbing material (usually cadmium) are inserted between the uranium containers to slow down the process to a manageable level. The radioactive decay generates heat, this creates steam that turns a turbine, which generates electricity.
Nuclear fusion is the opposite process. In nuclear fusion larger atomic nuclei are built from small pieces. This process is hard to manage because you need to input huge amounts of energy into the particles in order to force them to stick together. In the simplest case, two individual protons and two neutrons are joined together to form a helium nucleus. Note that protons do not want to stick together, as they both have positive charges, and positive charges repel each other ("electromagnetic repulsion"):
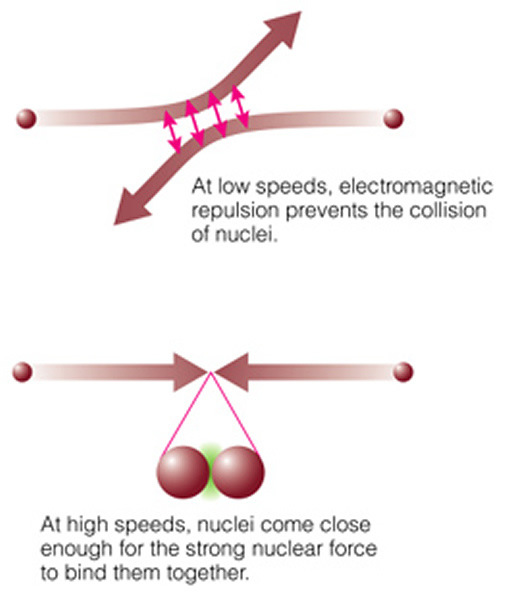
But if you can get two protons very close together, the strong nuclear force can overwhelm the electrostatic force, and the protons can stick together. The strong nuclear force is much, much stronger than the electrostatic force, but it only works over very short distances (while the electrostatic force works at ALL distances!). To get protons close enough together requires extremely high densities and temperatures--just like at the center of the Sun and the stars! The actual chain of events (the "proton-proton chain", see page 502) is shown here:
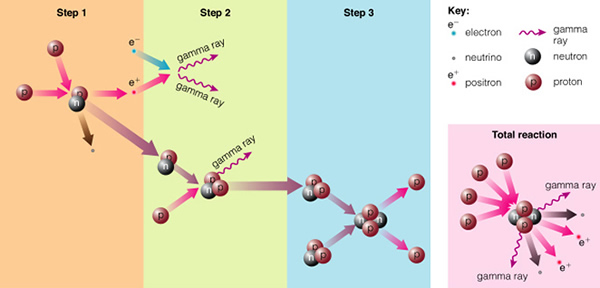
The energy released in the form of gamma-rays when two protons stick together ("step 1") act to heat the gas near the proton collision, and this in-turn heats the center of the star even more. This new nucleus, composed of one proton and one neutron (one of the protons changes to a neutron by emitting a positron), is the isotope of hydrogen called deuterium (2H). If we add another proton to this dueterium nucleus ("step 2"), we have the isotope of helium called "helium three" (3He). Normal helium is helium four (4He), containing two protons and two neutrons (shown in "step 3"). Add one more neutron, and you get helium four, a very stable nucleus (instead of just adding one more neutron, however, the more favored nuclear process is to ram two helium three nuclei together, in which helium four is produced, and two protons are expelled, as shown in the figure). In the Sun and most other stars, the hydrogen is being "burned" to produce helium. In the process, a large amount of energy is released, heating the core of the star to hotter, and hotter temperatures.
This is how the Sun and stars generate energy, the pressure at the center of the stars due to gravity generates high densities and temperatures. Gravity is relentless, constantly tugging on all the gas all of the time. Gravity tries to make the stars shrink. In the centers of stars, however, the temperature and density get so high, that protons can collide with other protons and stick together to form larger nuclei. This process releases energy, and fights gravity. We have a balance, the gravity tries to keep shrinking the star, but this shrinking generates heat, and eventually even greater amounts of energy via nuclear fusion. The heat generated in the center through nuclear fusion has to get out of the star---and in doing so it tries to make the star expand to lower density. Stars eventually reach an equilibrium size that depends on their mass, where the gravitational pressure and pressure produced by nuclear fusion are perfectly balanced.
We have now talked about light emission and absorption processes, but let's talk a little bit more about the interaction of light and matter and about how solar system objects look. The telescopes we talked about in a previous class were of two kinds: refractors and reflectors. Refractors bend light, reflectors use mirrors to alter the path of light:
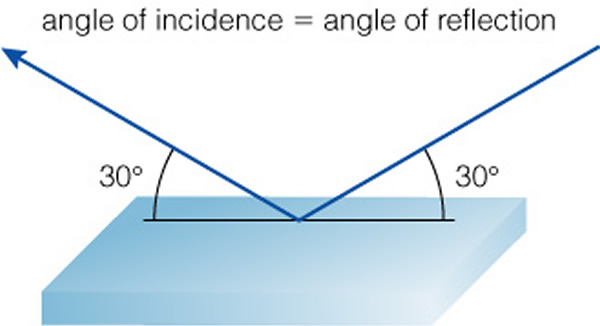
But all surfaces/objects reflect some amount of light---the floor, the walls, the table. And the angle which light is reflected, or scattered, can be different for different surfaces and therefore affect an object's appearance. Some surfaces are special, like a mirror, but so too is our projector screen, which is reflective, but one that "scatters" light in all directions:
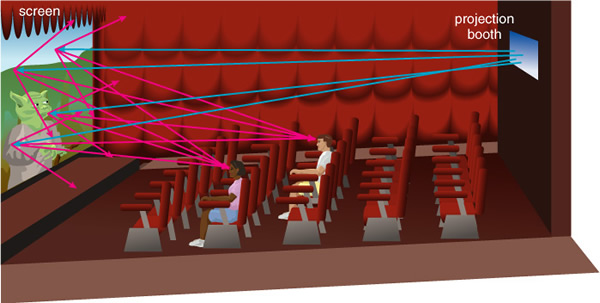
This allows you to view the image from different angles. For visual light, the light we can detect with our eyes, most substances merely reflect the light without changing its properties--or at least most wavelengths of light. In Figure 6.3 is a little cartoon showing how light reacts with matter:
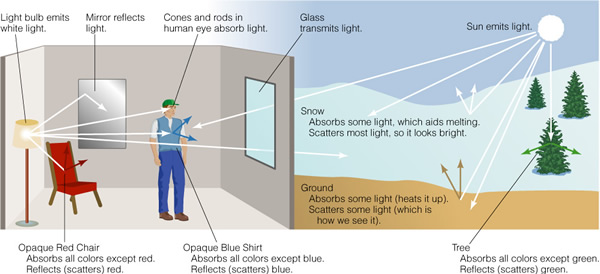
Red things look red because the reflect red light well, while absorbing all other colors. White things look white because the reflect all colors of light well. But what does this actually mean? There are four things that matter can do with light: 1) nothing (transmit it), 2) refract (bend) it, 3) absorb it, or 4) reflect/scatter it. Now, no object does any of these things perfectly, even for limited ranges of wavelength. For example, glass appears to be clear, but in fact all glass surfaces reflect some small amount of light. Normal glass reflects about 4% at its front surface and 4% at is back surface--thus it only transmits 92% of the light that hits it. This is due to the change in the "index of refraction" between the air and the glass. But even the best glass has imperfections (tiny bubbles and cracks), so there is also some scattering, reducing the amount of transmitted light further. "Anti-reflection" coatings are materials with a reduced index of refraction deposited on the glass surface and have an index of refraction between that of the glass (n = 1.5) and that of the air (n = 1.003). For limited wavelength ranges, you can improve the transmission rate significantly. Here is the reflectance curve of a standard coating on glass:
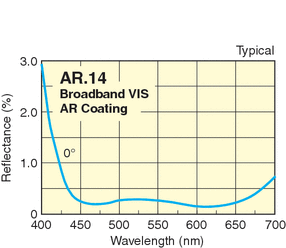
Note that this coating only works for limited range in wavelength, and starts reflecting in the blue---this is why many coated camera lenses appear bluish--they reflect more in the blue. The transmission and reflectance of light at optical surfaces is due to the wave nature of light, rather than how the electrons are arranged in the atoms of elements that comprise the glass. This is also true for the refraction that occurs when light enters a transmitting medium---in glass, light travels more slowly than in glass than in air (speed = c/n). This is how lenses bend light and form an image.
Why are some surfaces shiny, or mirror-like? Usually these are metals. Metals have large amounts of electrons that are loosely bound and can wander from atom to atom (that's why metals conduct electricity). These electrons can also reflect light--but how well a surface reflects light is a measure of how smooth the surface is. Mirrors are very smooth, so the light bounces backwards at the angle it arrived at ("angle of incidence = angle of reflection"). But a rougher surface (even if a shiny metal) looks duller because it scatters light in many directions:
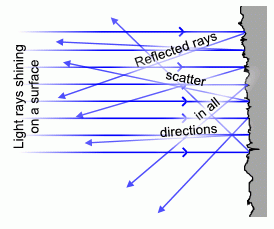
Ok, now we have covered scattering, we can answer the question "why is the sky blue". The sky is blue because air molecules scatter blue light better than red light---note that a typical atom is about the same size as the wavelength of (visible) light. The light interacts with the molecules of nitrogen and oxygen in our atmosphere, and scatter light in all directions. It is just that blue light is scattered more easily than red light (it contains more energy, and thus affects the molecules more than red light). If we could actually detect it, the sky would be violet, but our eyes don't have that type of response:
If the sky is blue, why are sunsets red? Because dust absorbs blue light better than red light, allowing it to pass, and that path at sunset is longer:
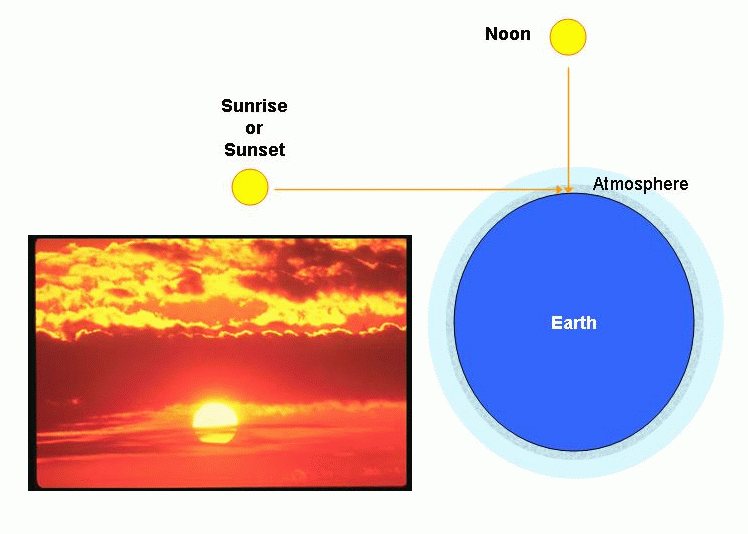
Also note that a sunset is generally redder than a sunrise---why? With daytime heating, more wind can be generated, and more dust lifted into the air. As the night cools, the winds will die off, and the dust will settle.
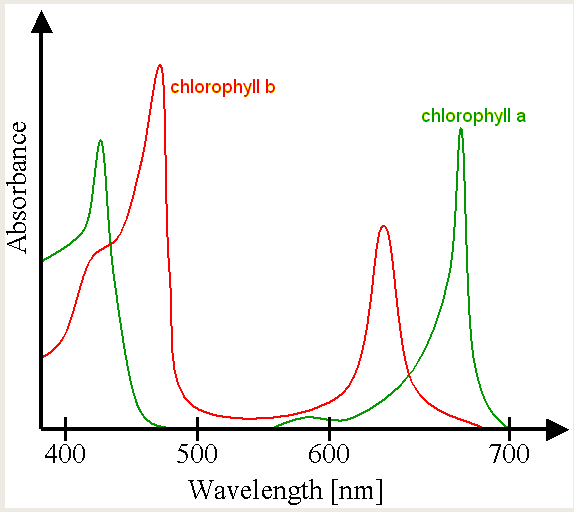
Ok, now lets look at why things have "colors". As noted above, things look blue or red because they preferentially reflect those colors, while absorbing others. Let's take plants--plants are green because the absorb the other colors, and reflect green. This is due to the chlorophyll molecule's spectrum:
Just like atoms, molecules have spectra. Whereas atoms have discrete energy levels for electrons, molecules are much more complicated, containing multiple elements that share electrons. And not only that, they bend and vibrate. Below are the emission line spectra of the element hydrogen, and the molecule H2:


All of the extra lines are just due to the fact that molecules can change their shapes, and this means electrons can move in complex ways.
So far we have really confined our discussion to the visible portion of the eletromagnetic spectrum. But objects emit a wide variety of radiation, and astronomers use all of these to understand the nature of the objects they study. Lets go back to figure 6.13 again:

All objects emit a spectrum that is characteristic of their temperature. In this plot, we have the spectra of three stars and a human being. Note the visible spectrum is a tiny, tiny piece of the electromagnetic spectrum. The shape of this curve, the "blackbody" spectrum, is a constant, and only the location of the peak changes with temperature. We see that the Sun has a temperature of 5800 K, and most of its light comes out in the visible part of the spectrum (that's why our eyes work there!). But the Sun emits light that is both bluer (higher energy) and redder (lower energy) than just the visible. Light that is too blue for our eyes to see is called "Ultraviolet", while light that is too red is called "Infrared". A very hot star emits more ultraviolet light, while a cool star emits more infrared light. Since planets, and humans, are much cooler than the coolest star, they emit all of their light in the infrared (except the reflected sunlight!!!). Everything in the Universe has a temperature, and therefore emits a spectrum that corresponds to that temperature. Let's take a look at the spectrum of Mars:
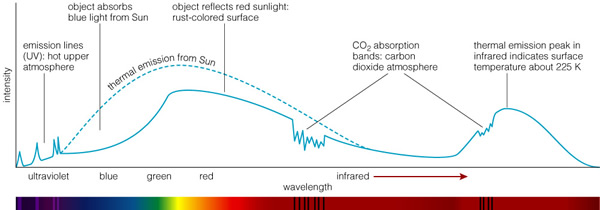
The dashed line shows the spectrum of the Sun (like that in the previous figure). Note that Mars, the "red planet", appears red because it reflects more red light than blue--it absorbs blue. But note that it isn't perfect, it absorbs some red light too. But note other features in the spectrum. In the near-infrared there are strong CO2 absorption features because Mars' atmosphere is almost pure CO2. Since the Sun emits some ultraviolet light, this can "ionize" atoms in the upper atmosphere, and these de-excite to emit UV lines. The heating of Mars means that the planet maintains a temperature very close to 225K, and thus it also emits a cool blackbody in the infrared. If you summed up all of these components, they would exactly equal that amount of thermal emission from the Sun that is intercepted by Mars (they have to since Mars does not generate its own heat anymore!). Here are infrared spectra of Venus, the Earth and Mars:
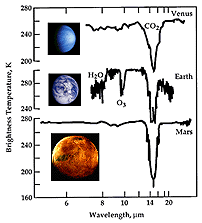
Note that the spectra of all three planets show the presence of CO2, but the Earth also has water absorption, and ozone (O3). Thus, spectroscopy at different wavelengths allows us to figure out both the temperature and composition of an object.
Infared: So let's examine how objects appear in the infrared. This is sometimes called "thermal imaging", as what we are detecting is the heat of an object/body. Here is an infrared image of a dog;
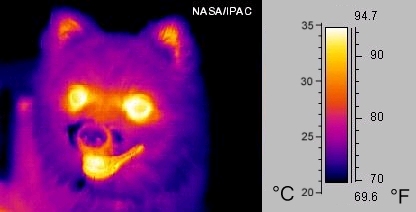
The yellows and whites indicate more light---the dog's mouth, eyes and ears are warm, and therefore "bright". But the dogs fur is cool, so it appears darker. Let's look at a comparison for Jupiter between visual and infrared:


The image in visible light shows us where there is strong reflection of the light of the Sun (white means higher reflectance), while the infrared radiation shows us where the heat from Jupiter is escaping (Jupiter generates a lot of internal heat). This allows us to understand the layers at which the clouds form, and how the circulation of the atmosphere is working.
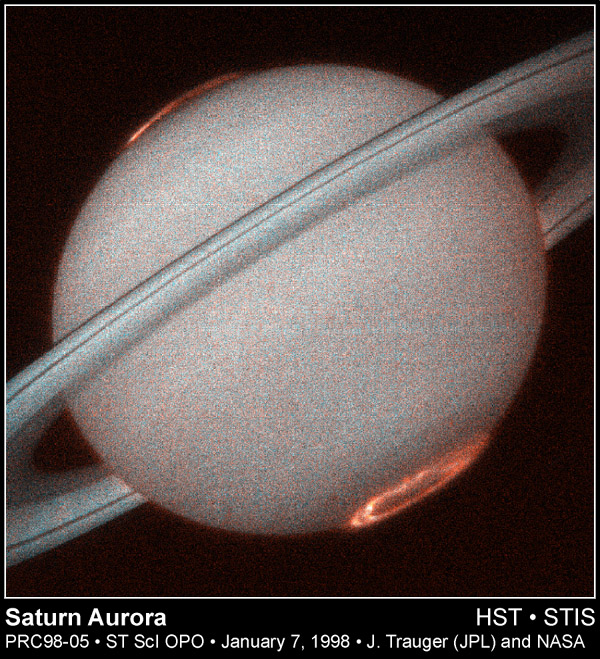
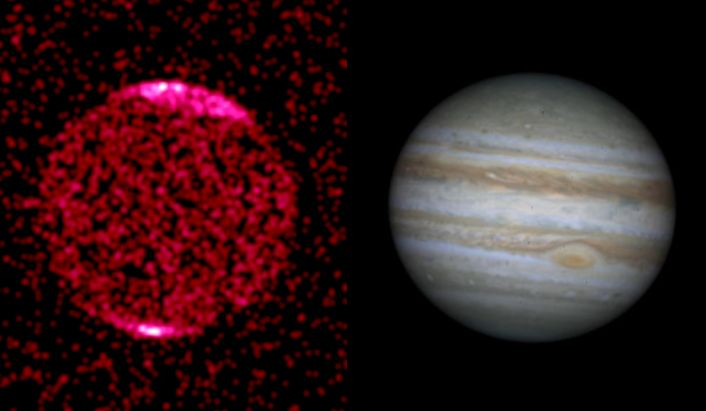
Ultraviolet: Just to the blue, or higher energy side of the visible light window is the ultraviolet. Some of you are familiar with ultraviolet light as the source of sunburns, or maybe from "black lights". These are the lowest energy photons of the ultraviolet. In contrast to infrared light, UV light carries a lot of energy, and thus can ionize atoms. Objects that emit a large amount of UV light typically have temperatures above 10,000 K. While most UV light is reflected from planets, some penetrates the atmosphere and creates zones of ionization--the "ionosphere" for example. The so called "ozone" (O3) layer of our atmosphere blocks most UV light from reaching the ground. Below is a picture of Jupiter in ultraviolet light which shows that most of the emission occurs near the planet's polar regions due to collisions between energetic particles and its atmosphere. These are the "aurorae" of Jupiter:

X-Rays: Beyond the ultraviolet, at higher energies we find X-rays. X-rays are so energetic they penetrate matter, and in the process can damage tissues, and other structures. But a number of objects in the solar system can be observed in X-rays--especially the Sun--and provide additional insight. One of the biggest surprises was that comets, as shown below

are detectable in X-rays. This is due to the interaction of the cometary gases with X-rays from the Sun. Very few planets actually emit X-rays, though the auroral regions of Jupiter have been observed:
Radio: At the other end of the energy range are radio observations. The processes that lead to radio emission are complicated, and we will not go into them here. But it is important to note that when magnetic fields are present, particles that get trapped in them emit radio waves. Thus, several planets are radio sources. For some images go here . We can also "image" some planets using radar. For example, the surface of Venus is obscured by thick clouds. Radar (radio waves!) can penetrate the clouds and reveal the surface. We will see numerous images of Venus that were made using radar (as shown below).
Here is a multiwavelength comparison for Venus (UV, Visible, IR, and radio):

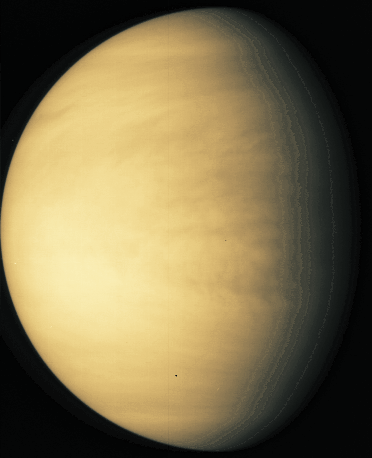
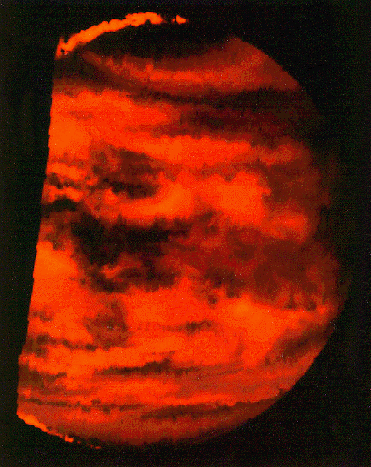
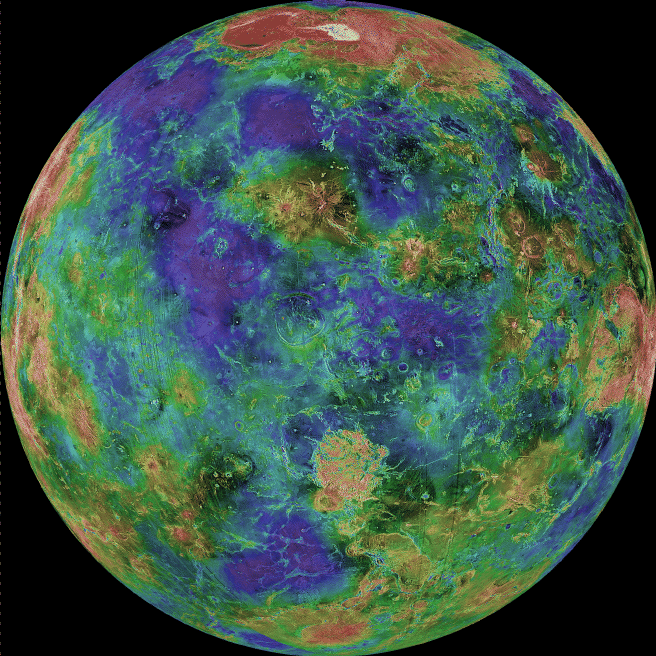
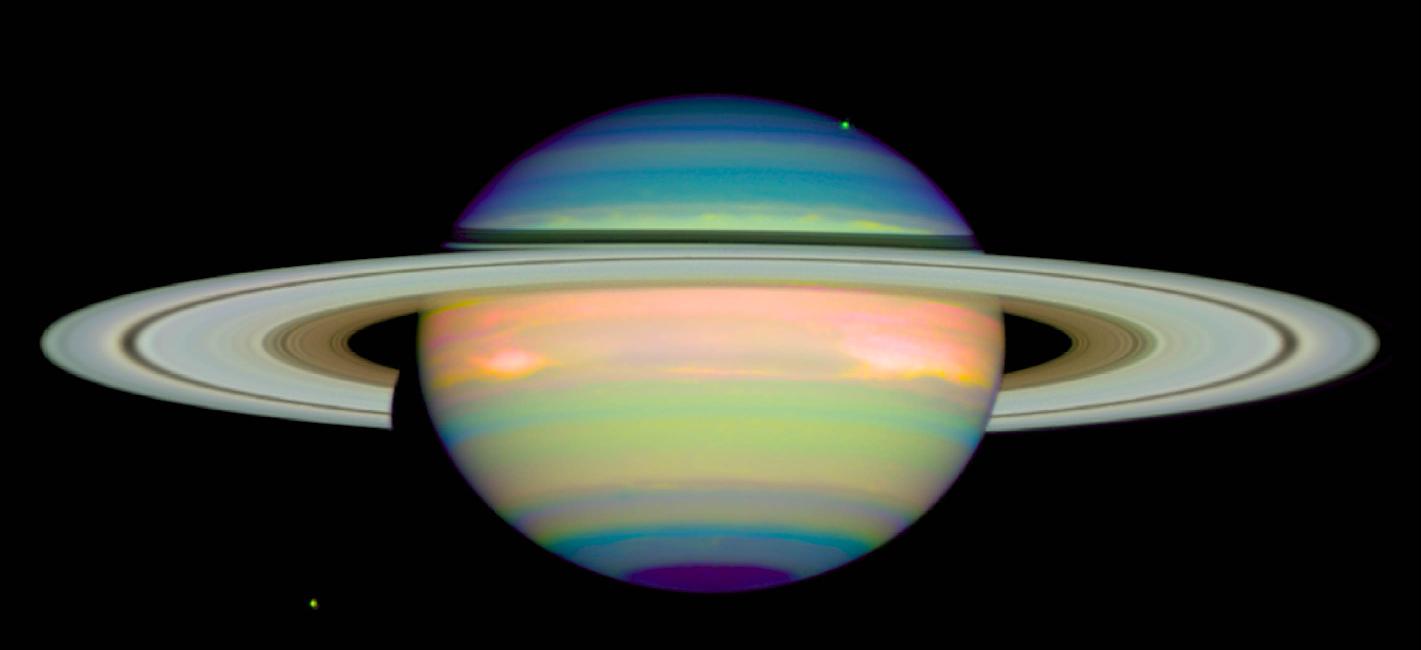
And here are UV, visible and IR images of Saturn: