Atomic Spectra- What Do We See From Atoms?
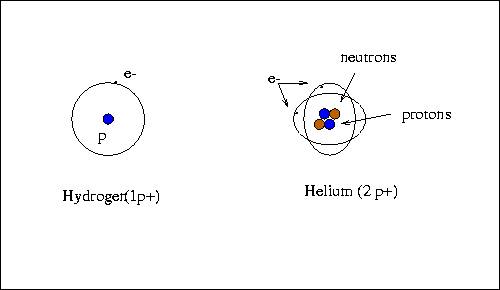
What makes up an atom? An atom is composed of a heavy nucleus of protons
(positively charged particles, written as p+) and neutrons (neutral
particles, written as no), around which orbit a cloud of extremely
light electrons (negatively charged particles, written as e-) .
What defines an element?
The number of protons in the nucleus of each
atom.
- Hydrogen (H) atoms have 1 proton.
- Hydrogen atoms with 1 proton and 1 electron are neutral
hydrogen (1H1).
- Hydrogen atoms with 1 proton, 1 electron, and 1 neutron are a heavy
isotope of hydrogen called deuterium (2H1).
- If a proton is added to hydrogen, we then have a different element
- helium (4He2).
- Nomenclature: For each element, the superscript denotes the number of
protons and neutrons, and the subscript the number of protons.
- How many neutrons are there in neutral carbon (12C6)?
There are a total of 12 nucleons (neutrons and protons),
and 6 protons, so there must be 12 - 6 = 6 neutrons.
- How many neutrons are there in the radioactive isotope
called carbon-14 (14C6)?
There are a total of 14 nucleons (neutrons and protons),
and 6 protons, so there must be 12 - 6 = 8 neutrons.
How does the make-up of the atom or element tell us what its spectrum will look like?
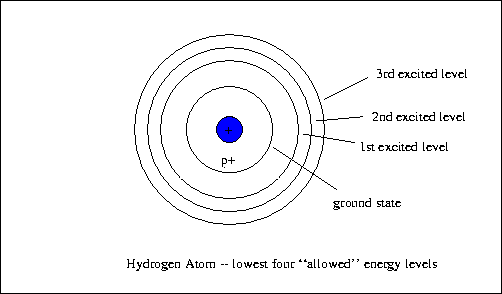
- Electrons exist in stationary states within atoms, each defined by
a discrete, unique level of energy. Only certain energy levels, like orbits
with certain radii, are allowed.
- Light, or radiation, emitted or absorbed by atoms as electrons move from
one energy level to another can be thought of as a stream of quanta
called photons. Each photon carries an energy E = h × v. We define
these energy levels as follows, saying that the electron is in an
excited state when it has extra energy (think of a child bouncing off
the walls with excitement).
- The ground state, the lowest energy level possible
- The 1st excited state, the next highest allowed energy level
- The 2nd excited state, the next highest allowed energy level
- The 3rd excited state, the next highest allowed energy level
- ...
- Till the point at which the electron is no longer bound to the atom
- An atom usually has the same number of protons and electrons. Because
protons have a positive charge and electrons have a negative charge, it carries
no charge in this state. When the atom loses (or gains) an electron we say
that it is ionized, and it then carries an electrical charge.
Entropy tells us that all things are naturally drawn to the lowest possible
energy state:
- Logs and water roll downhill.
- Bouncing balls slow to a halt.
- People collapse into bed at night and find it hard to get up in the morning.
- In the same fashion, hydrogen atoms tend to be in the ground state.
What
happens when we add energy to a Hydrogen atom, by bombarding it with photons?
- Most of the photons zip right past without interacting with the atom.
- But photons with just the right energy get absorbed by the atom.
- In this case, right means that the energy of the photon corresponds to
the energy level difference between allowed orbits in the hydrogen
atom, and absorbed means that the energy of the photon will be
taken into the atom (leaving the atom in a higher energy state).
- A photon with frequency v will be absorbed by an atom if the
energy of the photon corresponds to an energy level difference between allowed
states in the atom.
What happens next?
- Remember that entropy seeks the lowest available energy level for all
things, so the electron which has been raised to an excited orbit will
eventually drop back to the ground state.
- Conservation of Energy, tells us that the energy difference
between the excited state and ground state must appear somewhere when the
electron makes the transition. It is emitted by the atom as a photon, with the
same energy of the original one which was absorbed.
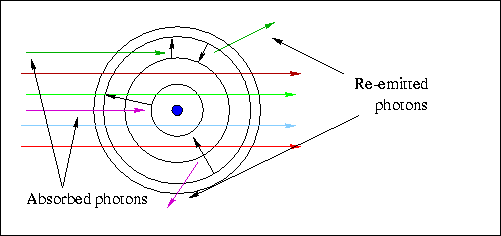
Here is a schematic diagram of the allowed orbits in a hydrogen atom. If
you can answer the questions listed below, you've got the right idea!
- Which transition(s) correspond(s) to the absorption of a photon?
A & D
- Which transition corresponds to the highest energy photon emitted ?
C
- Which transition corresponds to the shortest wavelength photon emitted?
C
- Which transition corresponds to the highest frequency photon emitted?
C