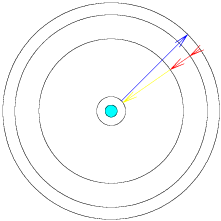
The diagram shown below represents the internal structure of an atom, in all of its glory. Atoms are much, much smaller than the objects which we normally encounter in our daily lives. Because of this, we may be quite surprised when we try to use our sense of intuition to predict how they should behave. (This just means that we expect things to work the way familiar things do, and are surprised when they follow different rules.)
We will use three analogies (comparisons) to describe the physics of atoms.
The Well
It can be helpful to visualize an atom as a deep well. In a
conventional well, water is drawn down toward the center of the Earth by the
force of gravity, and human effort is required to oppose this force and pull
the water up to the surface. In an atom, the electrons orbit the nucleus in a
series of energy levels. They are drawn toward the central nucleus by the
attraction between the positively charged nuclear protons and the negatively
charged orbiting electrons (remember that when it comes to the electrical
force, opposites attract). Energy is required to oppose this force, in order
for electrons to move up away from the nucleus. For an atom, this energy is
supplied not in the form of muscles, but in the form of light (photons).
In this analogy, the bottom of the well lies near to the nucleus of the atom, at the lowest (or ground) state of energy in which electrons can exist. Electrons located here are like frogs trapped at the bottom of a well. They would like to escape, but it takes a lot of energy to be able to climb up and out. This energy is supplied to frogs in the form of food, which in turn allows them to use their muscles to climb up the sides of the well.
For electrons the equivalent energy is supplied from incident photons. When a photon passes near to an electron, the electron absorbs the photon and gains the energy that the photon carried. The electron then uses this extra energy to jump from a lower energy level to a higher one, moving further away from the nucleus of the atom.

Once an electron has jumped to a high energy level, it does not stay there forever. Just as water flows downhill, and rocks roll down from mountain tops into valleys, electrons will fairly quickly drop down into any available lower energy levels. When they do this, they need to shed the extra energy that was keeping them at the higher level. They do this by emitting a new photon, which carries away the extra energy.
As electrons jump up and drop down from energy level to level, energy is neither created nor destroyed. This means that when an electron moves from level one to level two and then drops back from level two to level one, the amount of energy released by the electron as it falls back is exactly equal to the amount of energy which it absorbed when jumping up.
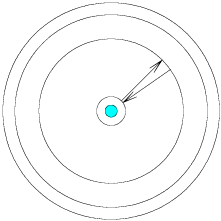
The Steps
When we described the atom as being similar to a well, the comparison seemed
to work quite well. However, on closer inspection we will see that the atomic
well differs from a conventional well in one very important aspect.
A frog climbing out of a well can move as much or as little as it chooses
it with each step, but an electron can only land at locations where an
orbital level exists.
It is as though the frog climbs up a smooth, continuous surface, while the
electron is faced with a ladder and can only jump from step to step. (Anyone
who has ever climbed up or down a ladder and missed a step knows that bad
things happen when you try to land on a non-existent step!)
We call the discrete (as opposed to continuous) energy levels of the atom a quantum process, because the energy required to move from level to level is quantized (occurring only in set sizes). Requiring that the energy levels be quantized is a lot like requiring that the frogs can only climb out of the well in one foot increments, and even if they tire they cannot take smaller steps.
Because the energy levels in an atom are quantized, the electrons are looking for photons which contain exactly enough energy (no more, and no less) to move them up from one level to a higher one. If a photon is available but it carries only half as much energy as is required to jump to the next highest level, the electron cannot absorb it and wait for a second one to contribute the missing energy. This photon will pass through the atom without interaction, and the electron will not shift levels until a photon with exactly enough energy is available.
The Rainbow
When we talk about atoms emitting or absorbing photons which are high or low
energy, it is important to connect the amount of photon energy to the
properties of the light. (Remember that a photon is simply an element, or a
piece, of light.)
A rainbow is composed of light which has been spread out according to energy level. Our eyes perceive the amount of energy carried by light as colour, for optical photons, and so when we see the many colours of the rainbow we are actually separating out photons of many energy levels, across the electromagnetic spectrum.
For optical light (light which falls in the range that can be seen with the human eye), the purple and blue colours correspond to high energy levels and the orange and red colour correspond to low energy levels.
| Low energy, Low frequency, Long wavelength |
 |
High energy, High frequency, Short wavelength |
When discussing the amount of energy in a photon, we can use three different words. Each word describes the same property of the photon.
We say that violet and blue light make up the high energy, high frequency, short wavelength end of the optical spectrum, while red light makes up the low energy, low frequency, long wavelength end.
For an electron to jump up a single energy level takes a small amount of energy. For the same electron to jump up several levels in one step takes a large amount of energy. If an electron needs to absorb a low energy, red light photon to move up one level, it should make sense that to jump up two levels at once the electron will need a higher energy photon, with colours closer to the higher end of the optical spectrum. If you can see that this means blue or violet coloured light, you have grasped the idea!
Similarly, if an electron absorbs a high energy photon (blue light) and jumps up several levels at once, but then drops down level by level and emits a series of photons, what colour will they be? Each will have a small portion of the energy absorbed originally, and so will have a redder colour than the original photon.