Spectra
The spectra observed from galaxies are formed from a combination of stars,
molecular clouds, and star-forming regions. We can examine them to determine
many properties, among them the radial velocity of the galaxy, the
star-formation rate, and the average age and metallicity of the stellar
populations, and the kinematics (mass) of the galaxy.
A spectrum has three components: the continuum, absorption lines, and emission
lines.
Continuum spectrum
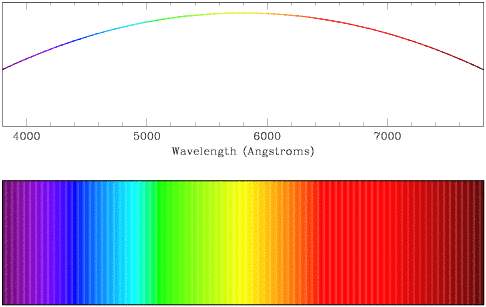
Continuous spectra (also called thermal or blackbody spectra) arise from
dense gases or solid objects which radiate heat. They emit radiation over a
broad range of wavelengths, thus the spectra appear smooth and continuous.
Stellar cores emit light in a predominantly continuous spectrum, as do
incandescent light bulbs, electric cooking stove burners, flames, fire
embers, and ... you.
| |
 |
| [NMSU, N. Vogt] |
|
Emission spectrum
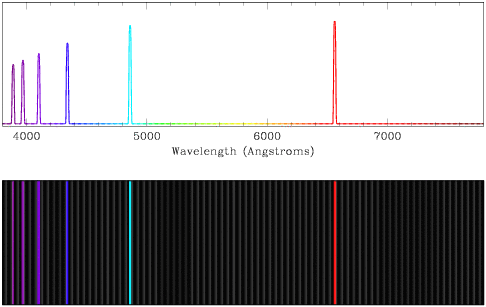
Discrete, non-continuous spectra are an observable result of the physics of
atoms. Unlike a continuous spectrum source, which can radiate at an arbitrary
frequency (just change the effective temperature), the electron clouds
surrounding the nuclei of atoms have very specific energies dictated by
quantum mechanics. Each element on the periodic table has its own set of
possible energy levels. Electrons tend to settle to the ground state, so an excited
atom with an electron in a higher energy level will emit a wave of light with
that exact energy to allow the electron to fall into the ground state. This
energy corresponds to a specific color, or wavelength, of light, so we see a
bright line at that exact wavelength. We can observe emission lines in
spectra from comets, nebula and certain types of stars.
| |
 |
| [NMSU, N. Vogt] |
|
Absorption spectrum
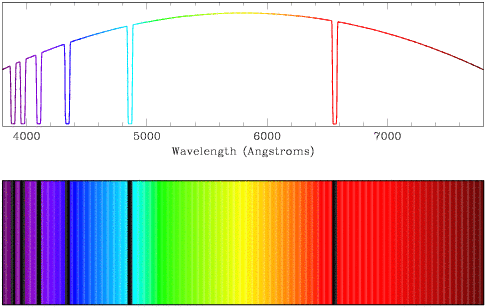
If light from a stellar core with a continuous spectrum encounters an atom,
the wavelengths corresponding to possible energy transitions within the atom
will be absorbed. The light may be re-emitted later, but as it will be
re-emitted in a random direction the spectrum along the line of sight will be
preferentially lacking in flux at the wavelength which corresponds to the
energy transitions within the atom. We can observe absorption features in
spectra from regions in space where a cooler gas lies between us and a hotter
source, from stars, from planets with atmospheres, and from galaxies.
| |
 |
| [NMSU, N. Vogt] |
|
Solar spectrum
This detailed spectrum shows the spectrum of the Sun, a nearly continuous
function broken by absorption features. Can you identify any of the species
present in the solar atmosphere, from the wavelengths or colours of the
absorption lines? What elements do you expect to feature prominently in
the solar spectrum?
 |
| [NASA/IPAC] |
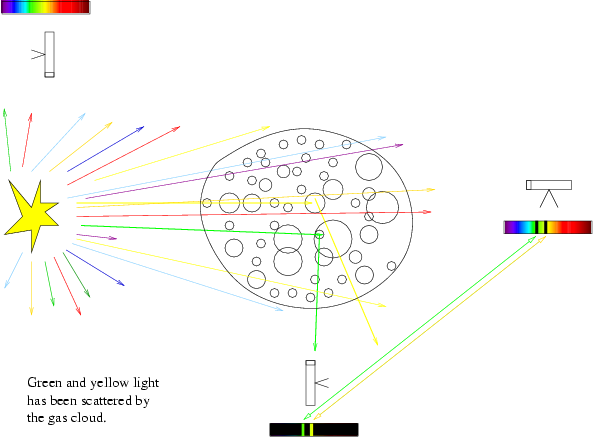
The diagram below shows how one could observe a continuum spectrum, and
emission spectrum, or an absorption spectrum from different positions around
a continuum source partially shrouded by a cool gas cloud.
 |
| [NMSU, N. Vogt] |




