 , and inversely
proportional to the period P or the wavelength
, and inversely
proportional to the period P or the wavelength  . We can show this mathematically, with constant h
(the amount of energy per unit frequency) and c (the speed of light).
. We can show this mathematically, with constant h
(the amount of energy per unit frequency) and c (the speed of light).
Summary of results:
Maria Mitchell's score on this quiz was 40% (any incorrect choices are highlighted in red).
#1. Consider two photons of light. Photon A has a frequency of 6 × 1014 Hz. if you know the of photon B, you know which photon contains more energy.
#2. The figure below shows a wave propagating in time. What is its frequency, in Hz (cycles per second)?
#3. A photon which shifts an electron from the first excited energy level to the ground state has energy than one which shifts an electron from the second excited energy level to the ground state (in the same atom).
#4. Is Heisenberg's uncertainty principle relevant to measurements of objects the size of an electron?
#5. How many different elements exist in nature?
Elapsed Time: 4.3 minutes
#1.
Consider two photons of light. Photon A has a frequency of 6 × 1014 Hz. if you know the of photon B, you know which photon contains more energy.| wavelength | period |
| frequency | wavelength, period, or frequency |
We know that the amount of energy E in a electromagnetic wave of light
is proportional to the frequency  , and inversely
proportional to the period P or the wavelength
, and inversely
proportional to the period P or the wavelength  . We can show this mathematically, with constant h
(the amount of energy per unit frequency) and c (the speed of light).
. We can show this mathematically, with constant h
(the amount of energy per unit frequency) and c (the speed of light).
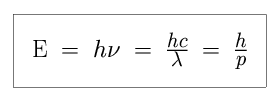
Shorter wavelength light contains more energy than longer wavelength light, which tells us that higher frequency light contains more energy than lower frequency light, and that shorter period light contains more energy than longer period light. If we know one of the wavelength, period, or frequency of a light wave, then we know the other two quantities, and the energy, as well!
Light with a wavelength of 3400 Angstroms has a period of 1.13 × 10-15 seconds, and a frequency of 8.82 × 1014 Hz.


#2.
The figure below shows a wave propagating in time. What is its frequency, in Hz (cycles per second)?| more | less |
| We need more information to answer this question. | |
The energy levels in an atom are arranged in ascending order, with the ground state being the lowest, followed by the first, second, third, and so on excited levels. An electron must always gain energy in order to move upwards, and always lose energy to move downwards.


#4.
Is Heisenberg's uncertainty principle relevant to measurements of objects the size of an electron?| Yes | No |
The uncertainty principle sets limits upon measuring the size and momentum (energy) that are relevant on the scale of subatomic particles. Objects the size of an atom or smaller can be affected.


#5.
How many different elements exist in nature?| three – hydrogen, helium, and the metals | roughly ten |
| roughly one hundred | roughly one thousand |
The heaviest element (the one with the most protons, and thus the highest atomic number) found in nature in sizable quantities is uranium, distinguished by 92 protons. Scientists have created heavier elements (with up to 115 protons) in laboratories, by smashing together two massive nuclei, but these artificial creations are always short-lived (they are stable for less than a second).

