Fission
Fission requires the presence of considerable unstable (radioactive) nuclei,
found in heavy, high atomic number elements. In a fission process, an
object is divided into parts (think of the word fissure, meaning a
gap). Remember that the atomic number (the number of protons in each nucleus,
defining its elemental composition) is written as a subscript for each atom in
our reaction, while the total number of protons and neutrons is written as a
superscript. In nuclear fission, a heavy atom can be divided into two atoms,
with atomic numbers that sum to equal the atomic number of the initial
radioactive atom. A few gamma rays (high-energy photons, designated by the
symbol 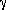 ) will be released in the process. Fission
requires the presence of substantial amounts of radioactive materials as
initial reactants, and as these materials are relatively rare in stars,
fission does not product as much energy as fusion does for powering the Sun.
) will be released in the process. Fission
requires the presence of substantial amounts of radioactive materials as
initial reactants, and as these materials are relatively rare in stars,
fission does not product as much energy as fusion does for powering the Sun.
Fusion
Fusion requires lots of protons, or the nuclei of hydrogen atom without
companion electrons. The word fusion relates to the verb to
fuse, meaning to combine. In nuclear fusion via the proton-proton chain,
for example, four individual protons are combined within a stellar core to
form a helium atom. Two of the protons stay in their initial form, giving us
an atomic number of two (and thus defining the final element as helium). The
two remaining protons transform into neutrons, and two positrons (positively
charged electrons, designated as e![]() ) are released, so that charge is conserved. As
with the fission process, energy is released in the form of gamma-rays, and a
few neutrinos (
) are released, so that charge is conserved. As
with the fission process, energy is released in the form of gamma-rays, and a
few neutrinos ( ) are also produced.
) are also produced.