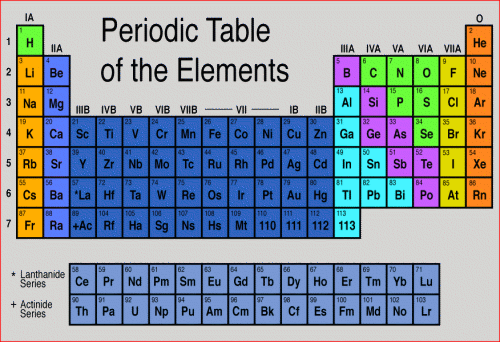

For example, hydrogen (H) is the first element listed and has an atomic number of 1. Each atom of hydrogen contains a single proton. Helium (He), with an atomic number of 2, has two protons, while lithium (Li) has three. Cesium (Ce), atomic number 58, has fifty-eight protons.
When an atom of any of these elements is in a neutral state, its positively charged protons are balanced by an equal number of negatively charged electrons. If an atom loses an electron, putting its proton and electron counts out of balance, it becomes ionized (and carries a net charge). The loss of the electron leaves it with an overall positive charge, but does not change its elemental identity.
If an atom gains or loses a proton, however, it becomes a different element. An atom of Cesium (Ce) that gains a proton becomes an atom of praseodymium (Pr), with 59 protons. It also becomes ionized, because the additional proton carries a charge of +1. Similarly, an atom of helium that loses a proton becomes an atom of hydrogen, with 1 proton, 2 electrons, and a net charge of -1.
Can you deduce from this which particle in an atom is crucial to its nature, and defines it?