The electromagnetic spectrum is just a convenient way to sort photons according to their energy. We have divided it into six broad regions. At the high-energy end of the spectrum, we have gamma-rays, often emitted in super-powerful astronomical gamma-ray bursts from distant galaxies, followed by x-rays (with enough energy to pass straight through normal human flesh), and then ultraviolet energy, able to burn your skin and sensitive components of your eyes. In the middle of the spectrum we find optical radiation, which illuminates our world at the wavelengths to which our eyes are most sensitive. At longer wavelengths we find infrared radiation (think of the glorious warmth of the Sun), followed by even lower energy radio waves.
As we shift from the high-energy end of the spectrum across to the low-energy regime, we also shift from short wavelengths to long wavelengths, from short periods to long periods, and from high frequencies to low frequencies. The common element is the speed, or velocity, with which these photons travel, no matter their energy. All of the gamma-rays and x-rays, the ultraviolet and optical photons, and the light emitted at infrared and radio wavelengths by the Sun take the same 8.3 minutes to travel from the Sun to the Earth. Photons of all energies which are released together will arrive together.

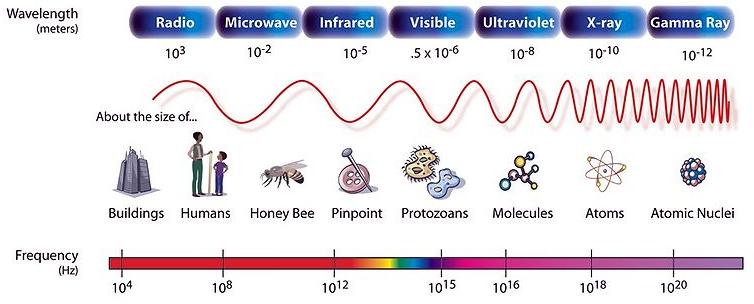
This NASA image shows the electromagnetic spectrum, sorted from low-energy photons on the left to high-energy on the right. The region between infrared and radio waves has been separated into a separate category, microwaves (this is the type of energy used in microwave ovens). The top label identifies the wavelengths of the light found within each broad category, with images of objects which are about the same size. What type of radiation is the same size (has a wavelength the same length as) an atom? What type of radiation is the same size (has a wavelength the same length as) a person? The bottom label notes the frequencies of the light, in units of Hertz (cycles per second).