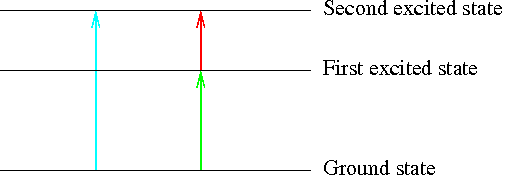
Our first sketch shows the energy levels in an atom. Remember that we often label the lowest energy level as the ground state (because you generally can't fall any lower than the ground), and then number the higher energy orbitals the first, second, and third, energy levels, and so on. It takes energy to ascend to each level in turn, just as if the electron were climbing a flight of stairs. The spacing of the energy levels within an atom is also essentially constant; it doesn't change from moment to moment.

Consider an electron which requires two packets of energy to shift from a low energy state to the next highest level (the ground state to the first excited state), and only one more packet of energy to shift up again to the next higher energy level (the second excited state). How many packets would it require to jump from the low energy level up to the final state, in one step?
It always takes energy to shift up to a higher energy level, and it requires the emission of energy to drop down to a lower energy level. The amount of energy required to jump up by two energy levels is equal to the amount of energy required to jump through the same gap, level by level.
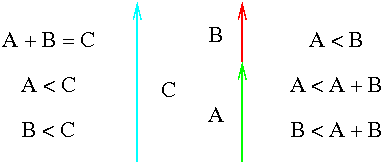
Consider this second sketch of three energy levels within an atom. The energy gaps between them are labeled A, B, and C, and are equivalent to three photons colored green, red, and blue. The green gap (A) is larger than the red gap (B), and both are smaller than their sum (C).