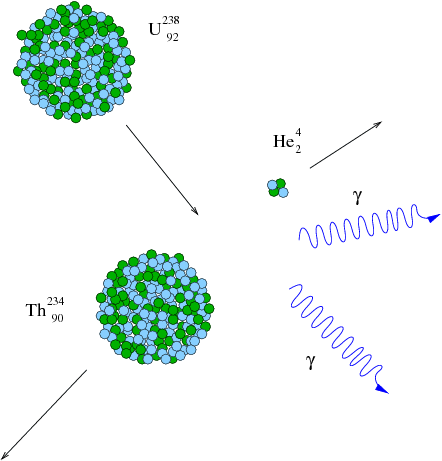
Certain heavy elements in the periodic table have nuclei that spontaneously break apart into pieces – these are the radioactive elements. If you weigh the initial nucleus and then weigh the pieces left after radioactive fission occurs, you will discover something amazing – they don't match.
 |
| [NMSU, N. Vogt] |
The missing mass in the fission reaction has been converted into energy via the well-known equation
which tells us that Mass and Energy are interchangeable.
Take the mass difference between the initial uranium nucleus and the sum of the final thorium and helium nuclei, multiply by the speed of light squared, and you will find the amount of energy that was converted from mass into energy in the reaction (released in the form of high-energy gamma rays). We can write this as an equation:
 mc2
mc2
where  m is the
mass difference between the uranium nucleus and the fission product nuclei.
m is the
mass difference between the uranium nucleus and the fission product nuclei.
This is a very efficient source of energy. Some fission reactions release as
much as 10 ergs/gram
of fissionable material.
ergs/gram
of fissionable material.
The problem is that only a small fraction of the Sun is made up of radioactive materials, so the amount of energy released by fission is not sufficient to power the Sun.
Thanks to Mike Bolte (UC Santa Cruz) for the base contents of this slide.